Analytical Methods and Instrumentation for Sulfur Dioxide and Dimethyl Sulfide
Alan R. Bandy and Donald C. Thornton
Drexel University
Sulfur dioxide and DMS will be determined by isotope dilution quadrupole mass spectrometry methods for the sulfur gases that were perfected at Drexel during the past twelve years (CS2, Bandy et al., 1985; OCS, Lewin et al., 1987; SO2, Driedger et al., 1987; DMS, Thornton, et al., 1990, Bandy et al., 1992; Bandy et al., 1993). These species can be measured by GC/MS/ILS at one sample per five minutes with a lower limit of detection of about 1 pptv, a precision better than 5% above 20 pptv, and accuracy within 10%.
The main components of our GC/MS/ILS systems are ABB Extrel Mass Spectrometry quadruple mass spectrometers and a custom-built gas chromatographs. We have two systems that are suitable for these studies. One of these instruments, used on the PEM Tropics A missions was downsized so that it occupies about one half its original rack space and consumes considerably less 60 Hz power.
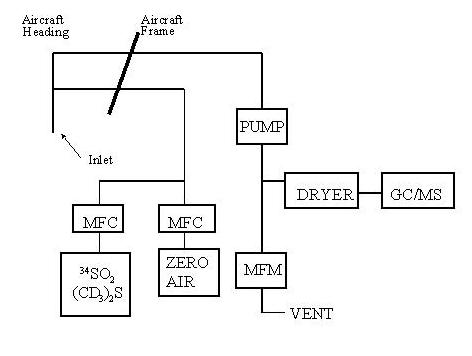
The sample manifold presently used for collecting and analyzing samples in real time is shown schematically in Figure 1.

The manifold, constructed from FEP Teflon tubing, draws ambient air into the aircraft by a Metal Bellows pump located near the instrument. By operating the manifold at about 3 psi with respect to absolute pressure, any leak which occurs after the pump is from inside the manifold to the ambient side. This insures that the sample collection is not contaminated by air where the instrument is located. The manifold airflow ranges from 10 to 60 L min-1 and is monitored by a mass flow meter, and the flow is exhausted through a needle valve, which is used to control the manifold pressure.
The manifold flow is sampled by another Teflon manifold at a flow rate of 200 to 1200 mL min-1. This air is dried by a Nafion dryer and then is passed though a Teflon trap cooled by liquid argon that removes the sulfur gases from the air stream. A mass flow meter is used to monitor the trap flow rate. Air passing through the mass flow meter is passed through tubing coaxial with the Nafion dryer to the trap pump. Trap contents are then volatilized with hot water and analyzed. Pretraps to remove ozone and other oxidants are required in the DMS analysis.
Calibrations are carried out by continuous standard addition of the isotopically labeled analyte to ambient air at a point very near the inlet of the Teflon manifold. Thus, losses in the manifold or instrument affect the standard and ambient analyte proportionately. Therefore, losses have no effect on the accuracy of the method unless they are so large that the detection limit is approached. Since the MS can separately and rapidly monitor the labeled (standard) and unlabeled (atmospheric) analyte, a standard addition calibration is included in every sample, thereby eliminating the need for a separate calibration sequence. Using this approach, very precise determinations of analyte can be made.
When sampling speed is not critical the pump can be placed after the point where the manifold is sampled to reduce the risk that the analyte would be removed in the pump and the risk that non-ambient analyte may enter the manifold through leaks in the pump. Well maintained pumps do not have these problems, but pump maintenance in the field is an unnecessary burden. .
Molecular ions of the ambient analyte and isotopically labeled standard analyte will be monitored for both DMS and SO2. In each case an algorithm is used which corrects for the isotopic abundances of the measured ions. The development of these algorithms is described in the literature (CS2, Bandy et al., 1985; OCS, Lewin et al., 1987; SO2, Driedger et al., 1987; DMS, Thornton, et al., 1990, Bandy et al., 1992, Bandy et al., 1993).
References
Bandy, A.R., Thornton, D.C., Driedger III, A.R., Airborne Measurements of Sulfur Dioxide, Dimethyl Sulfide, Carbon Disulfide and Carbonyl Sulfide by Isotope Dilution Gas Chromatography / Mass Spectrometry, J. Geophys. Res., 98, 23423 - 23434, 1993.
Bandy, A.R., Thornton, D.C., Ridgeway, R.G. Jr., Blomquist, B.B., Key Sulfur-Containing Compounds in the Atmosphere and Ocean: Determination by Gas Chromatography-Mass Spectrometry and Isotopically Labeled Internal Standards in Isotope Effects in Gas-Phase Chemistry, Chapter 25, J.A. Kaye, ACS Press, 1992
Bandy, A.R., Thornton, D.C., Driedger III, A.R., Airborne Measurements of Sulfur Dioxide, Dimethyl Sulfide, Carbon Disulfide and Carbonyl Sulfide by Isotope Dilution Gas Chromatography / Mass Spectrometry, J. Geophys. Res., 98, 23423 - 23434, 1993.
Bandy, A.R., Tucker, B.J., and Maroulis, P.J., Determination of Part-Per-Trillion by Volume Levels of Atmospheric Carbon Disulfide by Gas Chromatography/Mass Spectrometry, Anal. Chem., 57, 1310, 1985.
Lewin, E.E., Lalevic, M., and Bandy, A.R., Determination of Atmospheric Carbonyl Sulfide by Isotope Dilution Gas Chromatography/Mass Spectrometry, Anal. Chem., 59, 1802-1805, 1987
Driedger, A.R., Thornton, D.C., Lalevic M., and Bandy, A.R., Determination of Part-per-Trillion Levels of Atmospheric Sulfur Dioxide by Isotope Dilution Gas Chromatography/Mass Spectrometry, Anal. Chem., 59, 1196-1200, 1987
Thornton, D.C., Driedger, A.R., III, and Bandy, A.R., Determination of Part-Per-Trillion Levels of Sulfur Dioxide in Humid Air, Anal. Chem., 58, 2710-2715, 1986.